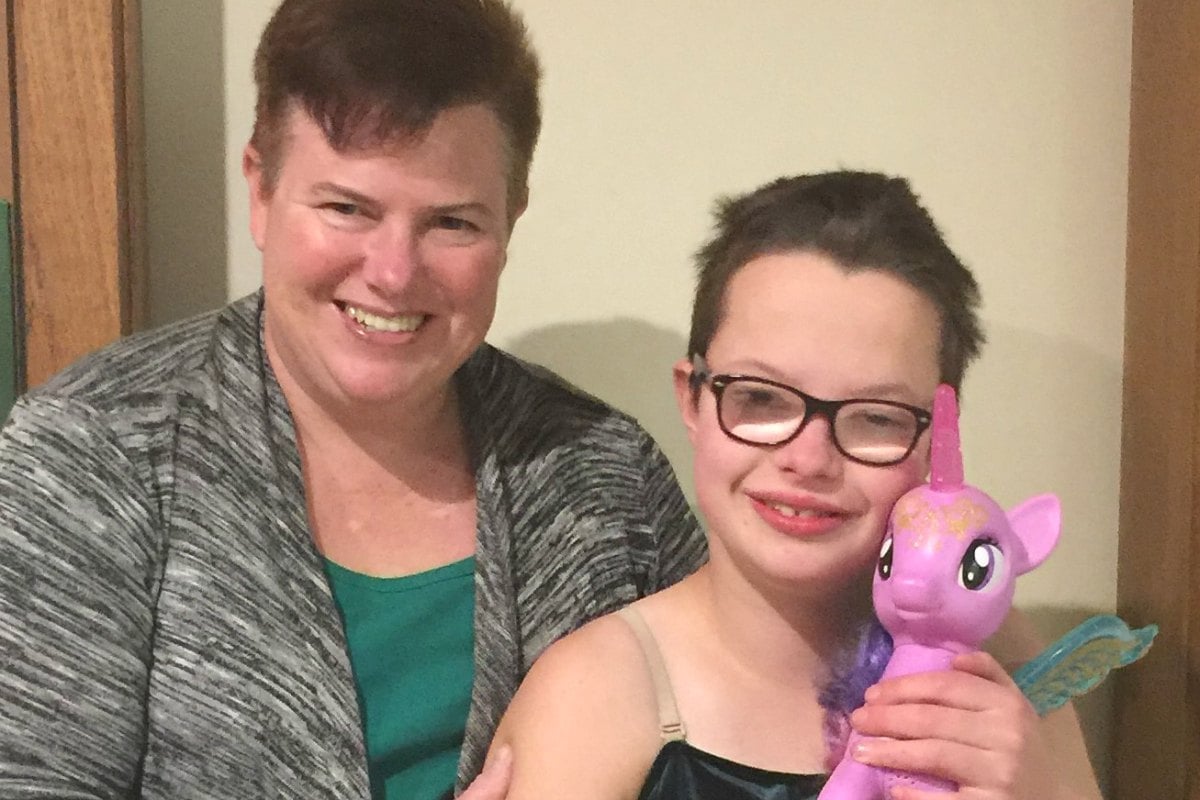
We celebrate when my 12-year-old daughter Rebecca puts her school uniform on without refusing, when she doesn’t swear at her teachers or peers, and doesn’t lift up her dress to seek attention.
A good day is when Rebecca doesn’t throw herself on the ground in a tantrum or say she won’t go to her weekly after school therapy sessions. Most mornings, I feel like I have run a marathon before we get out the door.
Rebecca is more than a handful and that is okay, I love my daughter, she is my shining light. I’ve learnt to adjust my barometer of what’s ‘normal’. Rebecca has a rare genetic condition called FOXP1 Syndrome which is neurodevelopmental and behavioural in nature.
It took us nine years before doctors confirmed Rebecca’s diagnosis. No parent should have to wait that long or longer, when we have genome sequencing testing which yields a 30-50 per cent diagnosis rate. The trouble is, the test is not funded by Medicare.




Top Comments
Good. It's about time people in this country had access to medicine that could help them manage their medical conditions.
First step towards accessing new medicines is to prove said new medicines actually work and are safe to use. Hence, clinical trials.
Cannabis has been used for thousands of years, both medically and recreationally. It seems to be an effective medicine for quite a few different medical conditions. It's also quite safe, no-one has ever died from a cannabis overdose, although they might have raided the servo for chips and soft drink.
Uh, no. It's not good enough to say it "seems to be effective" - you need high-level scientific proof that it *is* effective. Having a feeling that something might work, based on nothing more than anecdotal evidence, is a primitive, low-level approach to modern medicine. The safety profile of medications - ascertained by trials - means ascertaining evaluating optimum dosing, pharmacokinetics, pharmacodynamics and any interactions a new drug has with other medications and physical states. This also addresses a drug's toxicity profile and how best to manage side effects. Again, all done in clinical trials.
Lots of potentially harmful, but largely ineffective remedies have been "used for thousands of years". Historical habit is no substitute for scientific enquiry. You don't get to cut corners if you want to be intelligent and evidence-based in medicine.
Since it’s already been approved in the states, it seems a bit ridiculous that we need to conduct our own set of clinical trials. Talk about duplication of effort.